XRF- X Ray Spectrometer Gold Nugget Reading with Periodic Table Guide
- In stock, ready to ship
- Inventory on the way
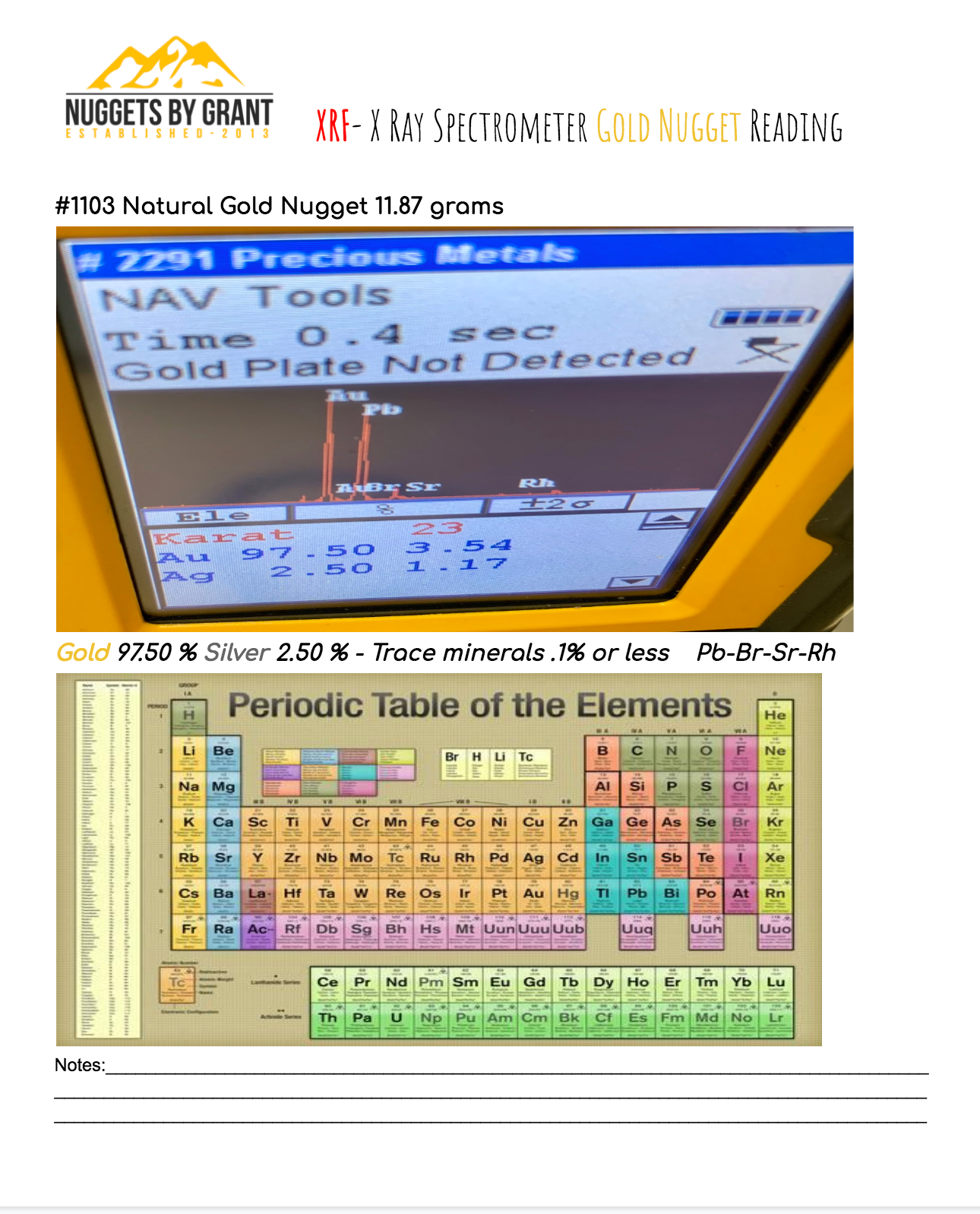
XRF- X Ray Spectrometer Gold Nugget Reading
Now XRF come with the Photo Scan only.
Check Out PHOTO #1 for Example:
Attention: This is a reading for Only my individual items not on Flake Gold or other items. If you buy more then 1 individual nugget then you must add more then 1 reading for $14.99 in the cart for each Gold Nugget that will be analyzed. Add in shipping notes the 1 to 4 digit # of which item you want the reading done to if you end up buying multiple nuggets but only would like one of them testes.
Contact me If you would like to have your other Nuggets analyzed then you must buy additional shipping.
What is XRF?
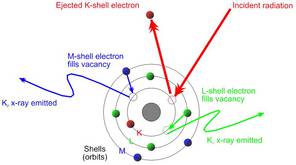
XRF is an acronym for x-ray fluorescence, a process whereby electrons are displaced from their atomic orbital positions, releasing a burst of energy that is characteristic of a specific element. This release of energy is then registered by the detector in then XRF instrument, which in turn categorizes the energies by element. Here is a detailed breakdown of the process:
- An x-ray beam with enough energy to affect the electrons in the inner shells of the atoms in a sample is created by an x-ray tube inside the handheld analyzer. The x-ray beam is then emitted from the front end of the handheld XRF analyzer.
- The x-ray beam then interacts with the atoms in the sample by displacing electrons from the inner orbital shells of the atom. This displacement occurs as a result of the difference in energy between the primary x-ray beam emitted from the analyzer and the binding energy that holds electrons in their proper orbits; the displacement happens when the x-ray beam energy is higher than the binding energy of the electrons with which it interacts. Electrons are fixed at specific energies in their positions in an atom, and this determines their orbits. Additionally, the spacing between the orbital shells of an atom is unique to the atoms of each element, so an atom of potassium (K) has different spacing between its electron shells than an atom of gold (Au), or silver (Ag), etc.
-
- When electrons are knocked out of their orbit, they leave behind vacancies, making the atom unstable. The atom must immediately correct the instability by filling the vacancies that the displaced electrons left behind. Those vacancies can be filled from higher orbits that move down to a lower orbit where a vacancy exits. For example, if an electron is displaced from the innermost shell of the atom (the one closest to the nucleus), an electron from the next shell up can move down to fill the vacancy. This is fluorescence.
- Electrons have higher binding energies the further they are from the nucleus of the atom. Therefore, an electron loses some energy when it drops from a higher electron shell to an electron shell closer to the nucleus. The amount of energy lost is equivalent to the difference in energy between the two electron shells, which is determined by the distance between them. The distance between the two orbital shells is unique to each element, as mentioned above.
- The energy lost can be used to identify the element from which it emanates, because the amount of energy lost in the fluorescence process is unique to each element. The individual fluorescent energies detected are specific to the elements that are present in the sample. In order to determine the quantity of each element present, the proportion in which the individual energies appear can be calculated by the instrument or by other software.
The entire fluorescence process occurs in small factions of a second. A measurement using this process and a modern handheld XRF gun can be made in a matter of seconds. The actual time required for a measurement will depend on the nature of the sample and the levels of interest. High percentage levels will take a few seconds while part-per-million levels will take a few minutes.